Technologies
(SIS) products
a highly regulated industry that ensures a high level of consumer and product safety.
SIS material is obtained from the intestine in a manner that removes all viable cells, but leaves the naturally fibrous and porous nature of the matrix behind.11 These proprietary processing methods retain the complex architecture and composition of the extracellular matrix, providing not only the structural collagen framework but also the natural non-collagenous ECM components that are essential for cell interaction, function, and ingrowth.11,12
SIS provides a natural scaffold that allows the body to restore itself through site-specific tissue remodeling.
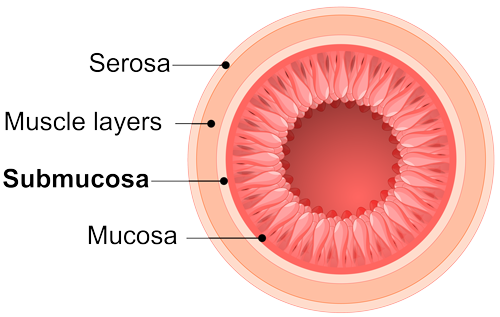
Tissue Remodeling
the renewal of tissue composition, and the reinforcement of structural tissue architecture.13
Recruit
Renew
Reinforce
References
2. Daley WP, Peters SB, Larsen M. Extracellular matrix dynamics in development and regenerative medicine. J Cell Sci. 2008;121(Pt 3):255-264.
3. McPherson JM, Piez KA. Collagen in dermal wound repair. In: Clark RAF, Henson PM, eds. The molecular and cellular biology of wound repair. New York: Plenum Press: 1988:471-496.
4. Raman R, Sasisekharan V, Sasisekharan R. Structural insights into biological roles of protein-glycosaminoglycan interactions. Chem Biol. 2005;12(3):267-277.
5. Sottile J, Hocking DC. Fibronectin polymerization regulates the composition and stability of extracellular matrix fibrils and cell-matrix adhesion. Mol Biol Cell. 2002;13(10):3546-3559.
6. Kristensen JH, Thorlacius-Ussing J, Ronnow SR, Karsdal MA. Elastin. In: Karsdal MA ed. Biochemistry of Collagens, Laminins and Elastin. 2nd ed. London: Academic Press: 2019:265-273.
7. Almond A. Hyaluronan. Cell Mol Life Sci. 2007;64(13):1591-1596.
8. Rizzino A. Transforming growth factor-beta: multiple effects on cell differentiation and extracellular matrices. Dev Biol. 1988;130(2):411-422.
9. Sarrazin S, Lamanna WC, Esko JD. Heparan sulfate proteoglycans. Cold Spring Harb Perspect Biol. 2011;3(7). pii:a004952.
10. Stringer SE. The role of heparan sulphate proteoglycans in angiogenesis. Biochem Soc Trans. 2006;34(Pt 3):451-453.
12. Nihsen ES, Johnson CE, Hiles MC. Bioactivity of small intestinal submucosa and oxidized regenerated cellulose/collagen. Adv Skin Wound Care. 2008;21(10):479-486.
13. Turner NJ, Badylak SF. Biologic scaffolds for musculotendinous tissue repair. Eur Cell Mater. 2013;25:130-143.
14. Franklin ME Jr, Trevino JM, Portillo G, Vela I, Glass JL, Gonzalez JJ. The use of porcine small intestinal submucosa as a prosthetic material for laparoscopic hernia repair in infected and potentially contaminated field: a long term follow-up. Surg Endosc. 2008;22(9):1941-1946.
15. Stelly M, Stelly TC. Histology of CorMatrix bioscaffold 5 years after pericardial closure. Ann Thorac Surg. 2013;96(5):e127-e129.
16. Badylak S, Kokini K, Tullius B, Whitson B. Strength over time of a resorbable bioscaffold for body wall repair in a dog model. J Surg Res. 2001;99(2):282-287.
17. Badylak SF, Park K, Peppas N, McCabe G, Yoder M. Marrow-derived cells populate scaffolds composed of xenogeneic extracellular matrix. Exp Hematol. 2001;29(11):1310-1318.
18. Hodde J. Extracellular matrix as a bioactive material for soft tissue reconstruction. ANZ J Surg. 2006;76(12):1096-1100.
19. Badylak SF. The extracellular matrix as a scaffold for tissue reconstruction. Sem Cell Dev Biol. 2002;13(5):377-383.
